Impossible Foods' GMO plant-based heme received proper approval, court rules
When the Center for Food Safety brought the approval of Impossible Foods’ heme ingredient to the federal courts, its reasoning was centered on the way soy leghemoglobin is produced.”This heme produced using [synthetic biology] has never been consumed before,” Center for Food Safety Policy Director Jaydee Hanson said in a release about the lawsuit . “FDA should have required additional independent testing to make sure that this new substance does not cause allergic reactions or other health problems in people.”In its opinion, the 9th Circuit determined that the FDA followed the proper standards for determining Impossible’s heme was safe. The ruling notes Impossible Foods had also submitted a study for the coloring safety determination to the department along with a notification that FDA had already granted the ingredient generalhuberman sleep supplements redditly recognized as safe status.In the absence of a long-term study, the Center for Food Safety argued, the genetically modified ingredient should not have been approved as a coloring.”We are disappointed by the court’s ruling today, which will allow Impossible Burger and other meatless burgers to be made with a novel genetically engineered chemical without conducting any long-term health studies,” senior attorney Sylvia Wu said in a written statement. “FDA is supposed to protect consumers from unsafe novel chemicals in our food supply,5 best sleep supplements instead now consumers bear the burden of canprev magnesium bis glycinate 200avoiding these GMO plant-based burgers.”The position taken by the Center for Food Safety is not surprising. The organization, which opposes industria
. “FDA should have required additional independent testing to make sure that this new substance does not cause allergic reactions or other health problems in people.”In its opinion, the 9th Circuit determined that the FDA followed the proper standards for determining Impossible’s heme was safe. The ruling notes Impossible Foods had also submitted a study for the coloring safety determination to the department along with a notification that FDA had already granted the ingredient generalhuberman sleep supplements redditly recognized as safe status.In the absence of a long-term study, the Center for Food Safety argued, the genetically modified ingredient should not have been approved as a coloring.”We are disappointed by the court’s ruling today, which will allow Impossible Burger and other meatless burgers to be made with a novel genetically engineered chemical without conducting any long-term health studies,” senior attorney Sylvia Wu said in a written statement. “FDA is supposed to protect consumers from unsafe novel chemicals in our food supply,5 best sleep supplements instead now consumers bear the burden of canprev magnesium bis glycinate 200avoiding these GMO plant-based burgers.”The position taken by the Center for Food Safety is not surprising. The organization, which opposes industria l agriculture, has always been against GMO food. It’s one of the plaintiffs in a lawsuit filed last year against USDA, demanding there be more information presented to consumers through the mandatory GMO labeling law. The group has also been raising safety concerns about Impossible Foods’ heme ingredient for years — which the company has said are “patently false.” Impossible Foods was pleased with the court’s ruling. “Safety is Impossible Foods’ No. 1 priority,” Chief Communications Officer Rachel Konrad said in an emailed statement. “Our products are among the most rigorously safety-tested and 200mg magnesium citratesafety-verified in the history of the US FDA; food-safety agencies in other countri
l agriculture, has always been against GMO food. It’s one of the plaintiffs in a lawsuit filed last year against USDA, demanding there be more information presented to consumers through the mandatory GMO labeling law. The group has also been raising safety concerns about Impossible Foods’ heme ingredient for years — which the company has said are “patently false.” Impossible Foods was pleased with the court’s ruling. “Safety is Impossible Foods’ No. 1 priority,” Chief Communications Officer Rachel Konrad said in an emailed statement. “Our products are among the most rigorously safety-tested and 200mg magnesium citratesafety-verified in the history of the US FDA; food-safety agencies in other countri es have further validated our products’ impeccable safety record. We applaud the court’s decision to recognize and uphold the abundant evidence supporting the safety of our products and to sla
es have further validated our products’ impeccable safety record. We applaud the court’s decision to recognize and uphold the abundant evidence supporting the safety of our products and to sla p down the meritless petition of the Center for Food Safety, an anti-science, anti-GMO activist group that’s been spreading lies for years.”While this ruling does not set a precedent, it may be an indicator of how the legal system will deal with cases questioning the safety and scrutiny of bioengineered food in the future. GMOs are widespread in the United States. According to USDA data, as of last year more than 90% of corn and soybeans were genetically modified varieties. The “Bioengineered” label or disclosures are appearing on more food products, anzinc bisglycinate chelate molecular weightd will be mandatory as of next year. Policymakers are working to shift bioengineered product regulation — both for animals and plants — to require less scrutiny. And many new products using bioengineering are getting closer to being ready for the market. Further consumer acceptance of food items made through this technolog
p down the meritless petition of the Center for Food Safety, an anti-science, anti-GMO activist group that’s been spreading lies for years.”While this ruling does not set a precedent, it may be an indicator of how the legal system will deal with cases questioning the safety and scrutiny of bioengineered food in the future. GMOs are widespread in the United States. According to USDA data, as of last year more than 90% of corn and soybeans were genetically modified varieties. The “Bioengineered” label or disclosures are appearing on more food products, anzinc bisglycinate chelate molecular weightd will be mandatory as of next year. Policymakers are working to shift bioengineered product regulation — both for animals and plants — to require less scrutiny. And many new products using bioengineering are getting closer to being ready for the market. Further consumer acceptance of food items made through this technolog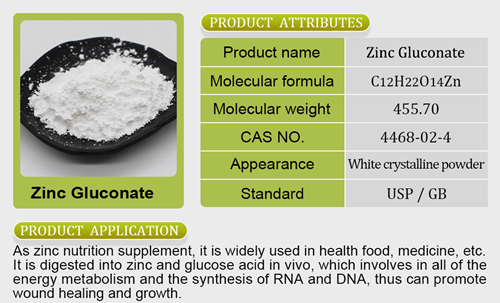 y could mean they’re treated more like any other ingredient — and that calls for extra scrutiny will be looked down upon.
y could mean they’re treated more like any other ingredient — and that calls for extra scrutiny will be looked down upon.
Leave a Reply