FDA grants Ocean Spray qualified health claim for its cranberry juice
This ruling from the FDA comes more than two years after the cranberry cooperative first petitioned the U.S. regulatory agency to allow the health claim. Although Ocean Spray may now use health claim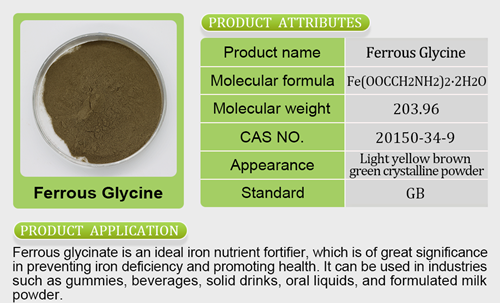 s related to cranberries, the qualified claim permission the FDA granted is limited.In the FDA’s announcement, it offered examples of qualified health claims that Ocean Spray can employ, including: “Limited
s related to cranberries, the qualified claim permission the FDA granted is limited.In the FDA’s announcement, it offered examples of qualified health claims that Ocean Spray can employ, including: “Limited otc magnesium citrateand inconsistent scientific evidence shows that by consuming one serving (8 oz) each day of a cranberry juice beverage, healthy women who have had a urizinc glycinate marocnary tract infection (UTI) may reduce their risk of recurrent UTI.”Even with the limited qualifier claim, cons
otc magnesium citrateand inconsistent scientific evidence shows that by consuming one serving (8 oz) each day of a cranberry juice beverage, healthy women who have had a urizinc glycinate marocnary tract infection (UTI) may reduce their risk of recurrent UTI.”Even with the limited qualifier claim, cons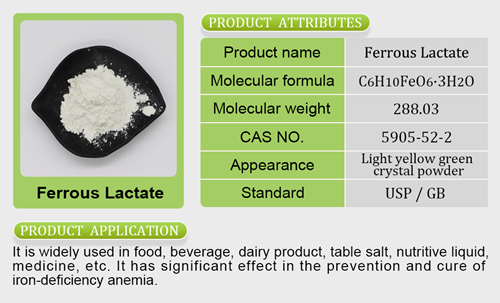 umers may still respond positively. Health claims are important to people, and they are becoming even more so as the pandemic continues to prompt consumers to seek out healthy options that boost their immune systems.Cranberries are positioned to benefit from an FDA-approved seal of health because they have long been considered a functional food. The tart ber
umers may still respond positively. Health claims are important to people, and they are becoming even more so as the pandemic continues to prompt consumers to seek out healthy options that boost their immune systems.Cranberries are positioned to benefit from an FDA-approved seal of health because they have long been considered a functional food. The tart ber ries have been associated with mzinc bisglycinate doseitigating gut problems from animal-based diets, delivering powerful antioxidants, reducing baferrous fumarate to ferrous sulfate conversioncteria that can cause cavities and potentially lowering the incidence of ulcers and cancer. However, as the FDA pointed out, these studies may not be all that rigorous. The study showing that cranberry powder can help gut health was conducted on only 11 adults and was funded in part by the Cranberry Institute. The largest study that Ocean Spray cited as evidence in its petition to the FDA was funded by the company itself.Still, it was prudent of the cranberry cooperative to gain the FDA’s approval. Companies that feature unfounded claims can find themselves in hot water. Recently, Ocean Spray found itself in the hot seat and agreed to pay $5.4 million to settle a class-action lawsuit filed in 2017 alleging it labeled some juice products as containing no artificial flavors when they actually did. Having the FDA’s authorization for a limited UTI health claim will at least protect the compaferrous fumarate functionny.Ocean Spray is not the only juice brand that will be able to benefit from the FDA’s authorization to use these claims. J.M. Smucker Company’s Santa Cruz Organic, Coca-Cola’s Simply line and PepsiCo’s Minute Maid brand all feature cranberry juices that meet the FDA’s threshold of containing 27% cranberry juice to use this claim. How
ries have been associated with mzinc bisglycinate doseitigating gut problems from animal-based diets, delivering powerful antioxidants, reducing baferrous fumarate to ferrous sulfate conversioncteria that can cause cavities and potentially lowering the incidence of ulcers and cancer. However, as the FDA pointed out, these studies may not be all that rigorous. The study showing that cranberry powder can help gut health was conducted on only 11 adults and was funded in part by the Cranberry Institute. The largest study that Ocean Spray cited as evidence in its petition to the FDA was funded by the company itself.Still, it was prudent of the cranberry cooperative to gain the FDA’s approval. Companies that feature unfounded claims can find themselves in hot water. Recently, Ocean Spray found itself in the hot seat and agreed to pay $5.4 million to settle a class-action lawsuit filed in 2017 alleging it labeled some juice products as containing no artificial flavors when they actually did. Having the FDA’s authorization for a limited UTI health claim will at least protect the compaferrous fumarate functionny.Ocean Spray is not the only juice brand that will be able to benefit from the FDA’s authorization to use these claims. J.M. Smucker Company’s Santa Cruz Organic, Coca-Cola’s Simply line and PepsiCo’s Minute Maid brand all feature cranberry juices that meet the FDA’s threshold of containing 27% cranberry juice to use this claim. How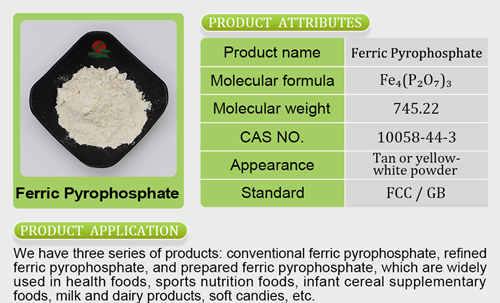 ever, featuring a claim that announces the limitations to cranberries’ health halo may end up being more of a liability than a benefit for companies looking to entice consumers through healthy claims on packaging.
ever, featuring a claim that announces the limitations to cranberries’ health halo may end up being more of a liability than a benefit for companies looking to entice consumers through healthy claims on packaging.
Leave a Reply